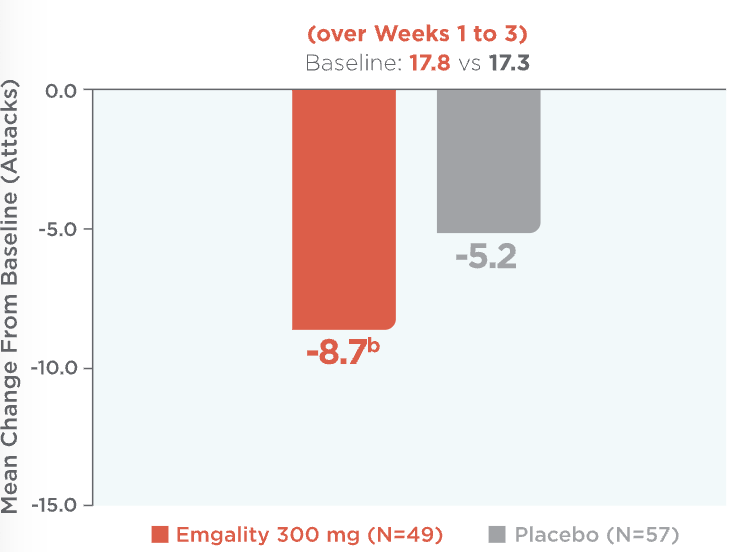
Emgality provided significant reductions in the average number of weekly cluster headache attacks: -8.7 with Emgality vs -5.2 with placebo (baseline: 17.8 vs 17.3)1a
Mean Reduction in Weekly Cluster Headache Attack Frequency (over Weeks 1 to 3)1,2a
aLS means are presented.2
bp=0.036 vs placebo.
LS=least square.
Mean reduction in weekly cluster headache attack frequency over Weeks 1 to 3 (baseline: 17.8 for Emgality vs 17.3 for placebo).1,2a Patients on Emgality 300 mg (N=49) experienced a mean reduction in weekly headache attack frequency of -8.7b vs -5.2 for placebo (N=57).
See study design for episodic cluster headache.
SELECT IMPORTANT SAFETY INFORMATION
Contraindications
Emgality is contraindicated in patients with serious hypersensitivity to galcanezumab-gnlm or to any of the excipients.
Emgality can make a difference as early as the first dose1,3,4
Mean Change in Weekly Cluster Headache Attack Frequency Over Weeks 1 to 31,3,4
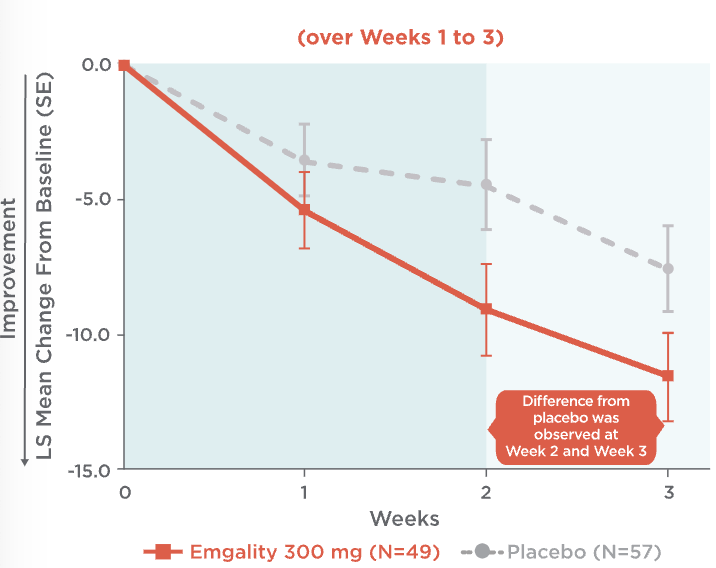
Mean change in weekly cluster headache attack frequency over Weeks 1 to 31,3,4:
- At Week 1, patients on Emgality 300 mg (N=49) experienced a mean change in baseline of -5.4 vs -3.6 on placebo (N=57)
- At Week 2, patients on Emgality 300 mg experienced a mean change in baseline of -9.1 vs -4.5 on placebo
- At Week 3, patients on Emgality 300 mg experienced a mean change in baseline of -11.6 vs -7.6 with placebo
Difference from placebo was observed at Week 2 and Week 3.
See study design for episodic cluster headache.
SELECT IMPORTANT SAFETY INFORMATION
Hypersensitivity Reactions
Hypersensitivity reactions, including dyspnea, urticaria, and rash, have occurred with Emgality in clinical studies and the postmarketing setting. Cases of anaphylaxis and angioedema have also been reported in the postmarketing setting. If a serious or severe hypersensitivity reaction occurs, discontinue administration of Emgality and initiate appropriate therapy. Hypersensitivity reactions can occur days after administration and may be prolonged.
For your patients with episodic cluster headache,
Emgality cut weekly cluster headache attacks in half or more for most patients at Week 31
71.4% of patients on Emgality 300 mg achieved a ≥50% reduction from baseline vs 52.6% of patients on placebo (p=0.046)1
Percentage of Patients Achieving ≥50% Reduction in
Weekly Cluster Headache Attack Frequency at Week 31
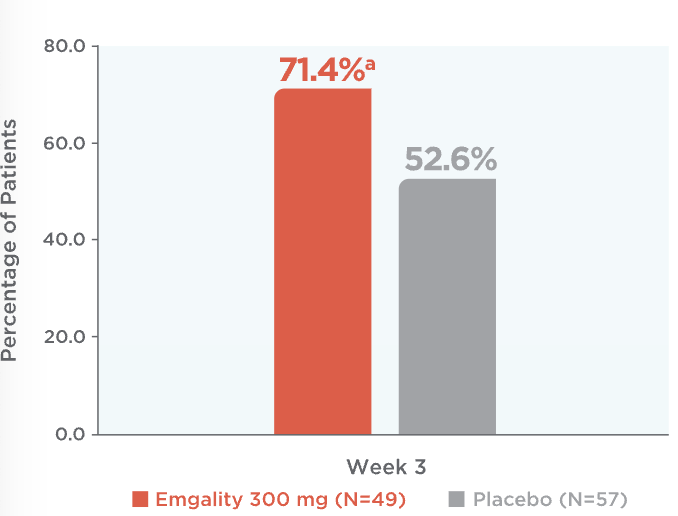
ap=0.046 vs placebo.
LS=least square.
At week 3, percentage of patients achieving ≥50% reduction in weekly cluster headache attack frequency on Emgality 300 mg (N=49) was 71.4%a vs 52.6% on placebo (N=57)1
See study design for episodic cluster headache.
SELECT IMPORTANT SAFETY INFORMATION
Adverse Reactions
The most common adverse reactions (incidence ≥2% and at least 2% greater than placebo) in Emgality clinical studies were injection site reactions.
References
- Emgality. Prescribing Information. Lilly USA, LLC.
- Data on File. Lilly USA, LLC. C-GZ-US-0120.
- Goadsby PJ, Dodick DW, Leone M, et al. Trial of galcanezumab in prevention of episodic cluster headache. N Engl J Med. 2019; 381(2):132-141.
- Data on File. Lilly USA, LLC. C-GZ-US-0093.